On October 7, 2020, the first webinar of the Virtual Medical Council (VMC), established by the WHO Regional Office for Europe with the support of the Center for Health Policy and Studies (Center PAS), within the framework of the regional TB-REP 2.0 project, took place. About 100 participants from different countries of Eastern Europe and Central Asia (EECA) joined the webinar.
The VMC began its works in September in parallel with the onset of the treatment for the first patients under the regional operational study on the introduction of modified short-term, completely non-injection MDR/RR-TB treatment regimens (as part of the European TB Research Initiative).
The webinar was opened by the Coordinator of the VMC, Dr. Elmira Gurbanova, Dr. Askar Yedilbaev, the Head of Tuberculosis Unit of the WHO Regional Office for Europe Joint Program on Tuberculosis, HIV and Viral Hepatitis and Mr. Oleksandr Korotich, the Research Consultant of the WHO Regional Office for Europe Joint Program on Tuberculosis, HIV and Viral Hepatitis.
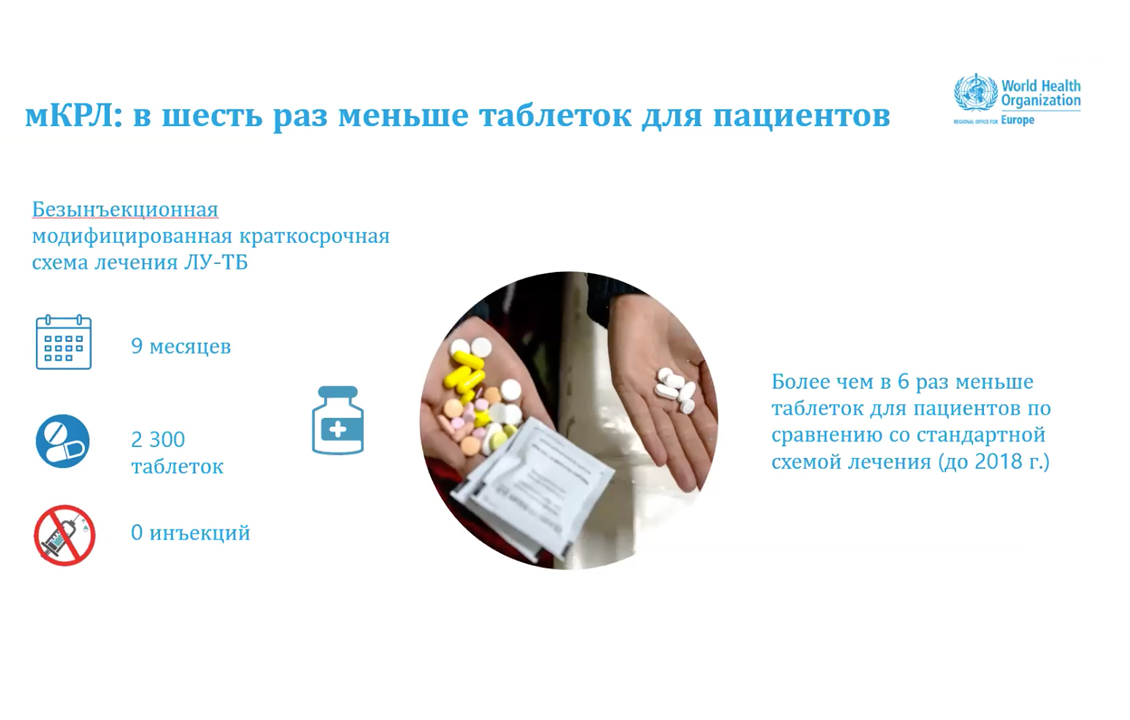
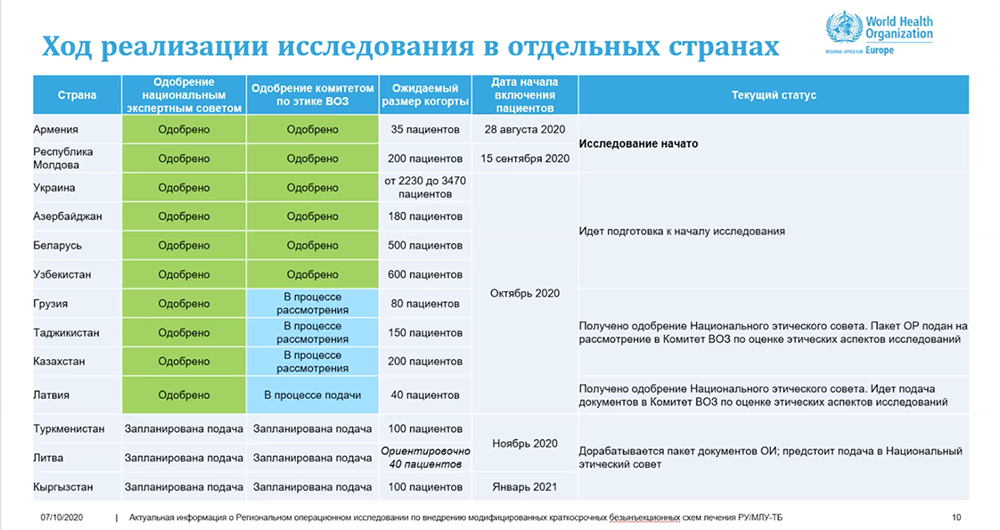
Thus, Dr. Edilbaev noted that at the moment this is a promising and unprecedented, in terms of patient cohort size and geographic coverage, operational study on DR-TB in the world, which in addition to improving patient treatment outcomes, will contribute to collection of a sufficient amount of data on the efficacy and safety of new treatment regimens for future WHO MDR-TB recommendations. Thirteen countries of the WHO European Region have already joined this initiative: Armenia, Azerbaijan, Belarus, Georgia, Kazakhstan, Kyrgyzstan, Latvia, Lithuania, Moldova, Tajikistan, Turkmenistan, Ukraine and Uzbekistan. Several countries are considering joining the regional initiative. Overall, it is planned that almost 6,000 MDR-TB patients from the listed countries will receive treatment with modified short regimens as part of the regional operational study.
Progress in the implementation of the regional operational study as of October 7, 2020
The new ERI-TB initiative seeks to: accelerate the introduction of ShoRRT MDR-TB treatment regimen in line with the latest WHO recommendations; facilitate the provision of appropriate clinical care to patients participating in the operational study; build and strengthen research capacity in the countries; expand global knowledge to create a new DR-TB policy guide. The WHO Office for Europe provides technical and methodological support to countries at all stages of OS through a working group and the Secretariat.
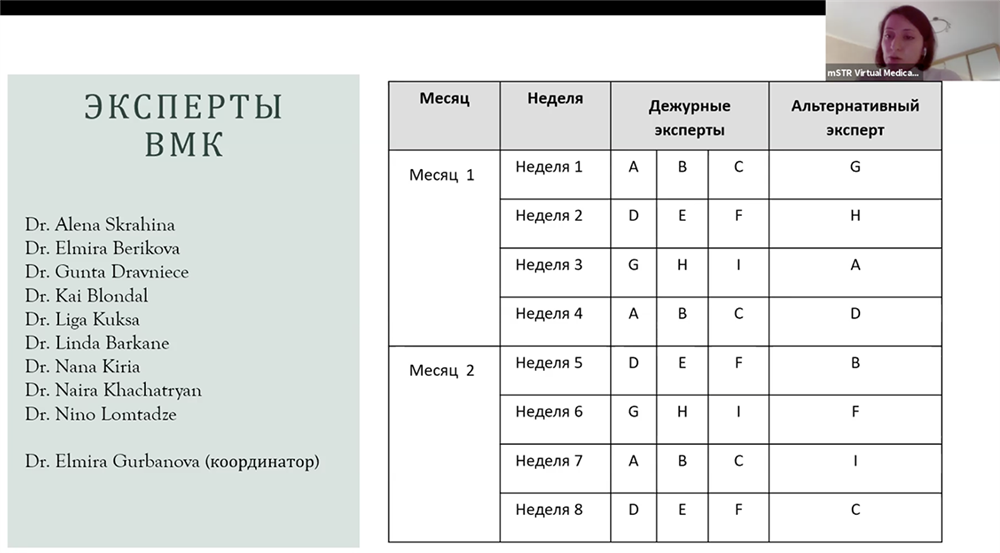
The Virtual Medical Council consists of 10 clinical experts, including the VMC Coordinator Dr. Elmira Gurbanova, who is responsible for shaping a consolidated collegial response in the country on behalf of the entire medical council. In her presentation at the online meeting, Dr. Gurbanova emphasized that the consultations and recommendations of the VMC experts will cover the following issues:
VMC support will be provided on a case-by-case basis in the form of expert response to inquiries submitted by individual clinicians and/or national medical councils. Written replies to the applicant will be sent within 48 hours from the date of request, excluding holidays and weekends.
In response to inquiries, the Council experts from different countries will propose the most appropriate and alternative therapeutic options for managing each individual case in accordance with the requirements of the operational study protocol and the latest WHO guidelines for TB treatment. The final answer of the VMC will be drawn up on the basis of consensus and quorum of three international experts.
Dr. Elmira Gurbanova noted that the VMC's answer is of a recommendation nature for each individual case and the final decision on the patient's treatment remains with the attending physician.
The forms for submitting a case to the VMC and the instructions for filing requests to the VMC are made available on the website: http://eurotb.net/ConsiliumForm.
Similar VMC webinars will be held at least once a month, according to Dr. Askar Edilbaev. They will deal with interesting and complex clinical cases, while maintaining the confidentiality of patient data. Further, the records of such meetings will be posted on the website with the support of the Center PAS for dissemination among a wider range of stakeholders.
As part of the current webinar, the options for choosing a treatment regimen and specifics of SHoRRT regimens were considered and thoroughly described by Dr. Gunta Dravniece, the expert of the VMC. Another topic of discussion was the doctor-patient interaction: informed consent and communication which was addressed by Dr. Liga Kuksha, an expert of the VMC. You can learn more about the presentations by accessing the webinar recording.
In order to make a request to the Virtual Medical Council (VMC), you should send a letter with the filled Case Submission Form (See "Filling out the Case Submission Form to the VMC") to the email: mstrconsilium@who.int